“Hedgehog” particles demonstrate new potential for green catalysis
Chemical Engineering-led research finds new potential for green catalysis using a unique property of hedgehog particles.

Chemical Engineering-led research finds new potential for green catalysis using a unique property of hedgehog particles.
A recent discovery from a University of Michigan research team led by Nicholas Kotov demonstrates new potential for nanostructured “hedgehog” particles for energy-saving biomimetic catalysis of high value molecules for chemical, polymer, and pharmaceutical industry.
The study published in Nature Communications focuses on solving high-energy consumption in the partial oxidation of hydrocarbons, and specifically cyclohexane, by taking advantage of the dispersibility of complex hedgehog particles in hydrophobic media.
“Green catalysis involves finding ways to produce everyday products and chemicals all around us that involve sustainable pathways,” co-first author and recent Michigan Chemical Engineering PhD graduate, Douglas Montjoy said. “It is crucial in the transition to new energy sources that we find ways to utilize renewable energy sources such as light to produce everyday products.”
“It is crucial in the transition to new energy sources that we find ways to utilize renewable energy sources such as light to produce everyday products.”
Douglas G. MontjoyMichigan Chemical Engineering Alum (PhD ’20)
Instead of gas phase oxygen, researchers used hydrogen peroxide (H2O2), a strong electron acceptor and “green” oxidant that produces water in the reaction. Unlike known pathways for the oxidation of cyclic hydrocarbons, the team observed the formation of valuable cyclohexene oxide used as an intermediate applicable to a wide array of products including epoxy resins and elastomers, pesticides, stabilizers for halogenated hydrocarbons and pharmaceuticals.
“We discovered a new pathway from the photo-oxidation of cyclohexane to the epoxide product,” co-first author and recent Michigan Chemical Engineering PhD graduate, Elizabeth Wilson said. “In particular, the direct epoxidation of any alkanes have not yet been reported, and we can show a direct link between this catalytic pathway and our unique catalyst particles.”
From a practical perspective, the exploration of the hedgehog particles as a catalyst for epoxide formation from alkanes rather than olefins has large implications, offering a remarkably lower economic cost as well as reduced environmental impact. The spiky catalytic particle can also be recovered and re-used reducing waste and pollution.
This epoxidation pathway could also lead to more efficient production of valuable chemical intermediates. “I think it is very exciting to see that nanoscale hedgehog particles enable a new chemical pathway to produce valuable intermediates,” Montjoy said. “In particular, the alkane to epoxidation chemical pathway enabled by hedgehog particles in this study has the potential to effectively reduce the CO2 emissions and the environmental impact of the traditional alkene to epoxidation pathway used in steam cracking and other processes today.”

Experiments in this study on catalyst design and characterization, product identification and quantification, and determination of reaction pathway were completed by Montjoy and Wilson as Chemical Engineering PhD students in the Kotov Lab.
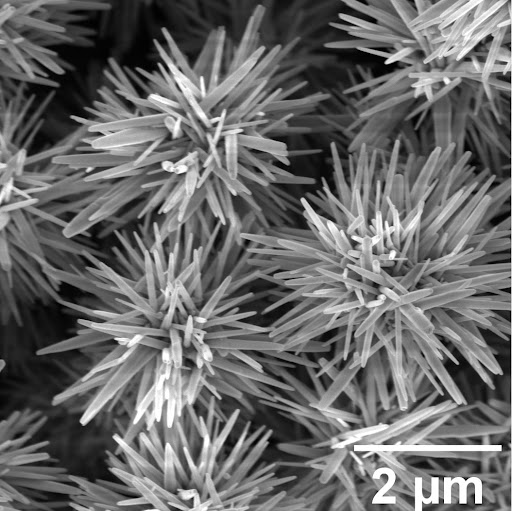
Created by a team of University of Michigan engineers, hedgehog particles are named for their bushy appearance under the electron microscope. Their development is detailed in a paper published in a 2015 issue of Nature, that showed they have remarkable ability to disperse in unfriendly solvents, such as hydrocarbons, where other particles clump together.
The topography of hedgehog particles replicates the geometry of pollen grains, ocean plankton, viruses and other examples of spiky particles found in different living organisms that take advantage of the same complex shape to reduce their clumping.
In all of these examples, decoration of the particle’s surfaces with spikes serves the same purpose of controlling agglomeration and attachment processes needed for the proliferation of the corresponding species.
Future research in this area will focus on control of selectivity through materials, oxidants and expansion to other alkane feedstocks and further explore how hedgehog particles can alter the spectrum making simple energy saving pathways to high value products.
“I hope these findings are able to expand research into nanostructured catalysts and alternative source materials for commodity products and chemicals,” Montjoy said.
Nick Kotov is Joseph B. and Florence V. Cejka Professor of Chemical Engineering and Irving Langmuir Distinguished Professor of Chemical Sciences and Engineering at the University of Michigan.