Crinkled coatings could prevent medical implants from failing
New bone cells and inflammation-reducing cells grab onto microscopic grooves in the coatings, stretching in ways that promote tissue healing.

New bone cells and inflammation-reducing cells grab onto microscopic grooves in the coatings, stretching in ways that promote tissue healing.
Written by Derek Smith.
Medical implants could fail less often when coated with a microscopically crinkled, ceramic material designed by researchers at the University of Michigan. The coating is described in a paper published in ACS Applied Materials and Interfaces.
The material’s very tiny crinkles are the perfect size for young bone cells and immune cells to latch onto. With their firmer grip on the coatings, human cells can strongly adhere to the implant and stretch out along its surface. Stretched bone cells develop faster, and stretched immune cells tend to help heal tissue and reduce inflammation rather than attack the implant as a foreign invader, so the researchers think their coatings could make medical implants more successful.
“Medical implants fail when they don’t integrate nicely with the body, either because immune cells try to repel the implant or there is inadequate tissue healing around the implant,” said Jouha Min, an assistant professor of chemical engineering and the corresponding author of the study.
“Most studies only focus on one problem at a time, but the coatings could tackle both problems and help a patient come back to their normal life much faster,” Min added.
When tissue around a medical implant doesn’t heal properly, the implant can loosen up and lose some function, which often requires corrective surgery. This issue, called aseptic loosening, accounts for around 20% of all hip revision surgeries and 25% of all knee revision surgeries, according to the American Academy of Orthopedic Surgeons’ 2023 report. Inflammation issues account for another 22% of hip revisions and around 33% of knee revisions.
Of the 3.1 million hip and knee replacements given between 2012 and 2022, nearly 236,000 required corrective surgery. With the yearly number of hip and knee replacements rising, lots of people stand to benefit from implants that integrate better with their bodies.
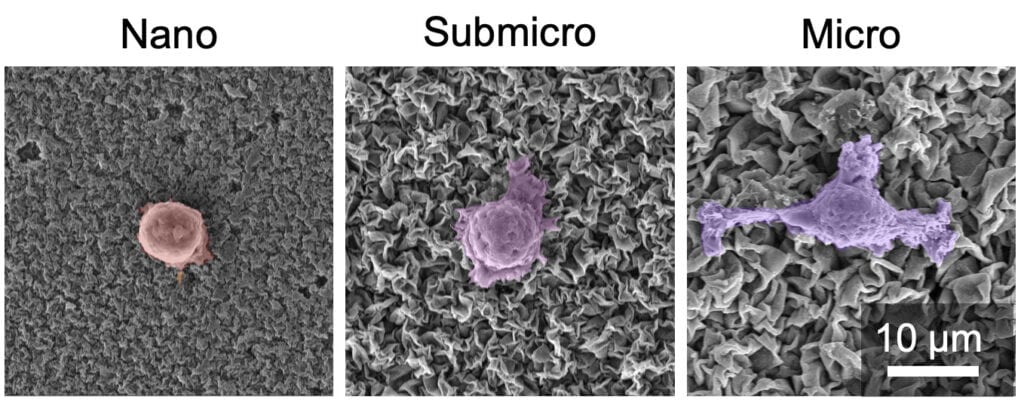
The crinkled coatings could help make a successful implant, but only if the size of the grooves in the coatings can be precisely controlled, according to the new study. When the grooves in the coating are around 2 micrometers wide, human cells start stretching and forming finger-like appendages to adhere to the grooves, which are more than 10 times smaller than the bone cells.

As the cells feel their way around their surroundings, they also turn on genetic switches that stimulate development into mature bone cells or immune cells that reduce inflammation. When the width of the coating’s grooves is smaller than one micrometer, the cells don’t stretch. As a result, bone cells develop more slowly, and immune cells are triggered to fight foreign bodies and cause inflammation.
“By going from the nano- to the microscale, we can direct the tissue cells in favor of a successful implant,” said Mohammad Asadi Tokmedash, a doctoral student of chemical engineering and the study’s first author.
The researchers’ new coating manufacturing method allows them to control the size of the grooves. They first add the ceramic material onto a sheet of polystyrene layer by layer. Once the ceramic is sufficiently thick, the researchers heat the polystyrene sheet, causing it to shrink. The attached ceramic shrinks with the polystyrene, but it also cracks and deforms because it is less flexible than the polystyrene. The resulting cracks and bends become the grooves that the cells latch onto, and the size of the grooves is controlled by the thickness of the ceramic layers added on top of the polystyrene sheet before it is heated.
There’s one downside to omitting smaller, nanoscale grooves from the coating—they are better at preventing pathogenic bacteria from adhering to it by creating a rough, spiky surface that damages bacteria cell membranes. Larger, microscale grooves may be ideal for human cells to latch onto, but they also provide lots of space for bacteria to hide in and grow on the coatings.
Because infection is another major cause of implant failure, the team is currently working on a method to allow their coatings to kill bacteria while also providing safe handles for human cells to attach to. Future experiments will test their idea.
The coatings were studied at the Michigan Center for Materials Characterization.
The research was funded by the University of Michigan, College of Engineering.
Jouha Min is also a professor of materials science and engineering.