Faster, more sensitive lung cancer detection from a blood draw
Capturing nanoscale ‘packages’ that cancer cells send out, twisting gold nanoparticles use light to distinguish healthy patients from lung cancer patients.

Capturing nanoscale ‘packages’ that cancer cells send out, twisting gold nanoparticles use light to distinguish healthy patients from lung cancer patients.
Written by Patricia DeLacey.
A new way of diagnosing lung cancer with a blood draw is 10 times faster and 14 times more sensitive than earlier methods, according to University of Michigan researchers. The microchip developed at U-M captures exosomes—tiny packages released by cells—from blood plasma to identify signs of lung cancer.
Once thought to be trash ejected from cells for cleanup, researchers discovered in the past decade that exosomes are tiny parcels containing proteins or DNA and RNA fragments that are valuable for communication between cells. Although healthy cell exosomes move important signals throughout the body, cancer cell exosomes can help tumors spread by preparing tissues to accept tumor cells before they arrive.
“Cancer exosomes leaving the tumor microenvironment go out and kind of prepare the soil. Later, the cancer cell seeds shed from the tumor and travel through the bloodstream to plant in the conditioned soil and start to grow,” said Sunitha Nagrath, a professor of chemical and biomedical engineering at U-M and co-corresponding author of the study in the journal Matter.
Exosomes carry proteins both inside the parcel and on their outside surface. Like many biological molecules, these surface proteins are chiral—meaning they have a right- or left-handed twist—which causes them to interact with light in unique ways.
In cancer exosomes, surface proteins are often mutated, meaning a genetic change altered the order of the molecules that make up the protein. Mutations subtly change the shape of the protein, which also shifts its chirality.
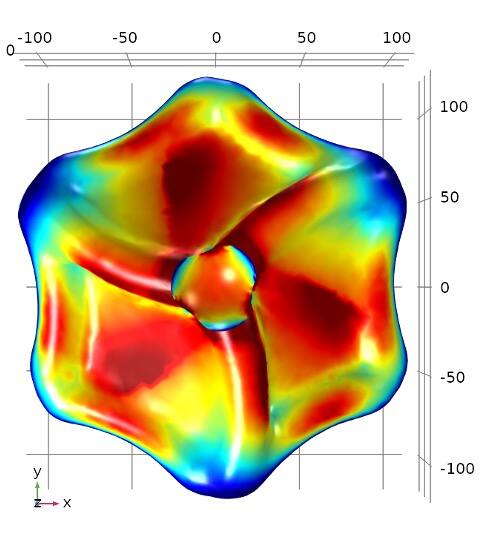
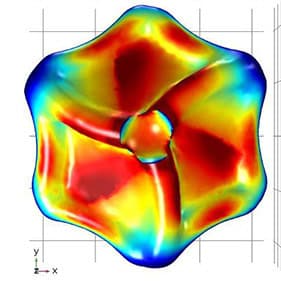
These differences can be spotted through interactions with twisted—or circularly polarized—light, which can match the twist in the protein. The resonance creates a strong signal returned to a light detector. However, these light signatures are typically weak and hard to interpret. Furthermore, exosomes must be extracted from a blood sample to do this kind of detection. This is tricky because exosomes are small—measuring just 30 to 200 nanometers (a millionth of a millimeter).
To spot them, the research team designed gold nanoparticles shaped like twisted disk (adapted from a structure first described in a 2022 Nature study) that capture exosomes in a central cavity. Because of a nearly perfect match in size, shape and surface chemistry, these cavities reliably catch exosomes.
With a right-handed twist, they resonate strongly with right-twisting light but don’t send back much signal if the incoming light has a left-handed twist. This different response to twisted light is known as circular dichroism. The proteins on the captured exosomes, sunk into the cavity, can strengthen or reduce the intensity of the return signal depending on their shapes. Studded along the tiny channels of a microfluidic chip, the gold cavities captured exosomes from blood plasma and revealed distinct signatures between samples given by healthy study participants and those with lung cancer.
“While I expected the optical activity of nanoparticles to be dependent on the mutations in the proteins, I was pleasantly surprised how sensitive it was. This is due to the fact that nanoparticles are all oriented in the same way in the detection device,” said Nicholas Kotov, the Irving Langmuir Distinguished University Professor of Chemical Sciences and Engineering at U-M and co-corresponding author of the study.
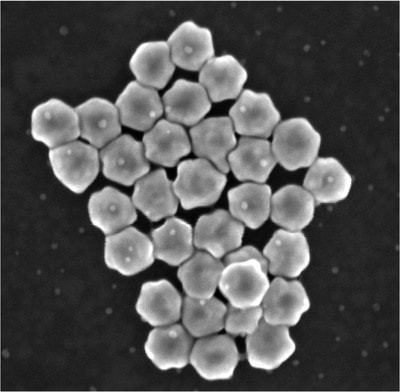
The microfluidic chips, named CDEXO chips for Circular Dichroism detection of EXOsomes, may be able to distinguish among specific lung cancer mutations, helping doctors make treatment decisions to target the dominant mutations as they change.
The researchers envision the CDEXO chip will first be used alongside traditional diagnostic methods. As trust in the technology develops, the chip could be used to screen for other cancers to improve early detection.
“As a next step, we want to look at most known solid tumor mutated proteins to understand how their spectral signatures are different. From here, we can push the technology to further increase those spectral differences to distinguish between proteins,” said Nagrath.
Kotov is also the Joseph B. and Florence V. Cejka Professor of Engineering and a professor of macromolecular science and engineering.
Sunitha Nagrath is also the co-director of the Liquid Biopsy Shared Resources of the University of Michigan Rogel Cancer Center.
This work was conducted at the Lurie Nanofabrication Facility and Michigan Center for Materials Characterization. Collaboration with the University of Michigan Rogel Cancer Center contributed both cell lines and clinical expertise.