Flexible DNA linkers enable “impossible” nanostructures
Nanoparticles that couldn’t fit together with conventional DNA “glue” may now be created with the help of joints added to the rigid DNA.

Nanoparticles that couldn’t fit together with conventional DNA “glue” may now be created with the help of joints added to the rigid DNA.
Written by Derek Smith in collaboration with Seth Zimmerman.
Using DNA to guide the self-assembly of nanoparticles has been limited by the stiffness of DNA molecules, but pliant spacers added to the DNA can enable previously impossible shape combinations, researchers from Northwestern University and the University of Michigan have shown.

Such materials may be designed to interact with light in new ways, potentially leading to technologies like “invisibility cloaks” and ultra high-speed optical computing systems, the researchers say.
“Creating structures from nanoparticles could lead to materials with interesting optical effects because the spacing between the particles can be at the scale of light waves,” said Sharon Glotzer, the Anthony C. Lembke Department Chair of Chemical Engineering and co-corresponding author of the study published today in Science.
“In most of the studies out there, there’s one particle shape that is linked together to make something cool. Now, we can start to think about mixing and matching different shapes,” added Glotzer.
The team’s flexible ligands significantly expand the design space for nanotechnology.
“We’ve enabled the creation of highly ordered colloidal crystals with shapes and sizes previously deemed impossible to make,” said Wenjie Zhou, a PhD graduate from Northwestern University and one of the study’s lead authors.
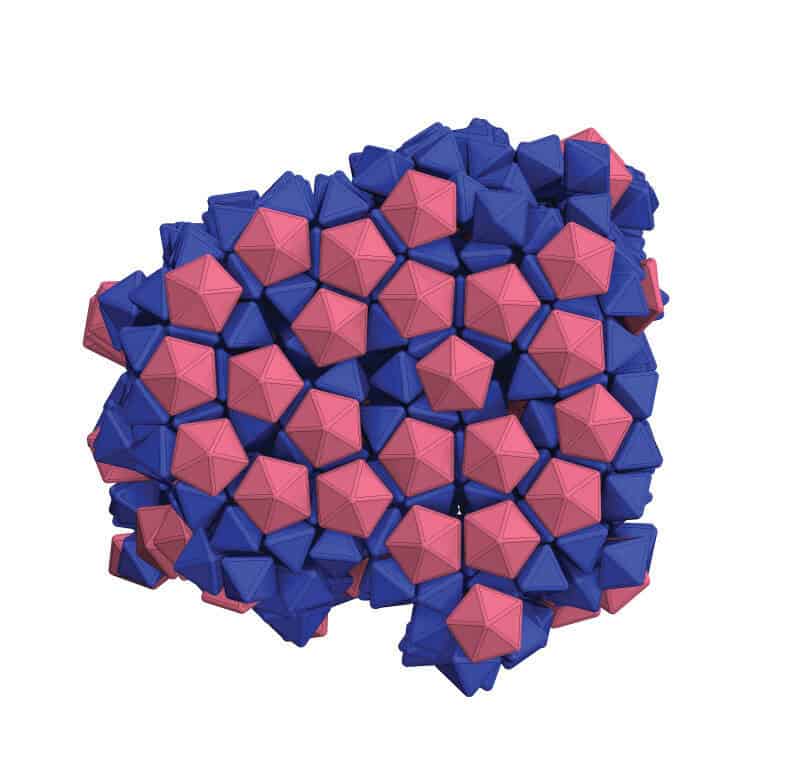
The team managed to pack together shapes that don’t normally fill space well and combine two variations on the octahedron (a particle with eight faces) into a honeycomb-like structure. Such feats bring the team closer to combining different nanoparticle properties in ways that currently don’t exist within a single material.
Nanoparticles are like bricks that engineers use to build larger structures, but these blocks are too small to be picked up and placed. Instead, engineers need to convince the particles to assemble themselves into the desired structures.
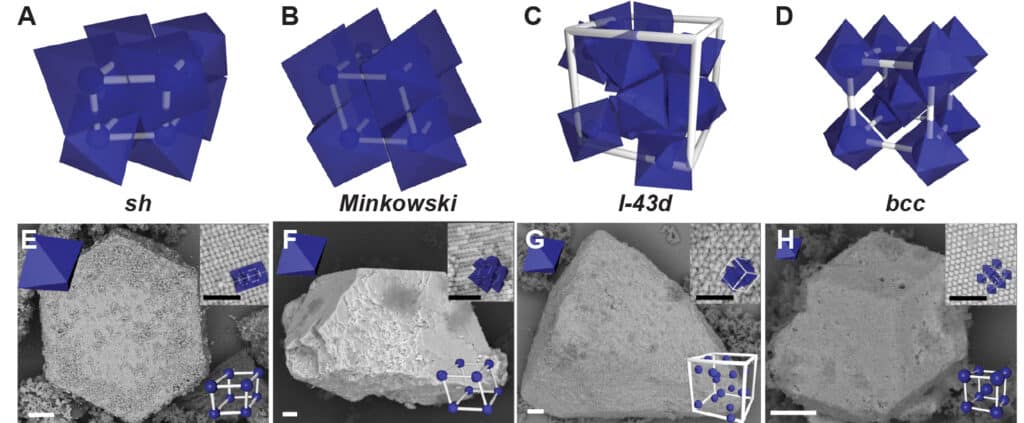
Because single strands of DNA bind only with complementary strands, Mirkin’s group has been using them as programmable adhesives that tell nanoparticles how to stick together. The problem is, DNA molecules are like stiff rods, and that prevents all of the DNA molecules from binding when the shapes of the nanoparticles prevent their faces from perfectly aligning.
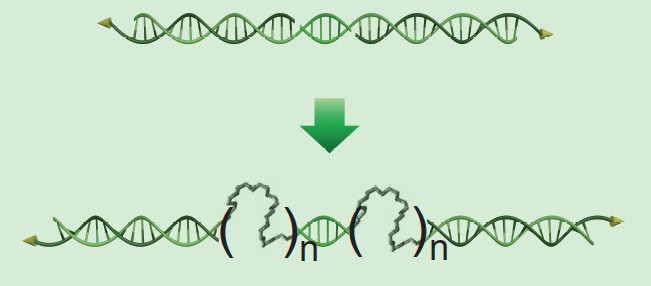
To overcome this downside, the team inserted flexible molecules into the DNA, which act like the accordion part of a bendy straw. This added flexibility allows the DNA to glue together nanoparticles that don’t perfectly align.
“DNA and nanoparticles have dimensions on the same length scale and we can chemically encode particles with DNA so they can be designed to recognize complementary particles, and therefore the DNA effectively becomes our hands,” said Chad Mirkin, the George B. Rathmann Professor of Chemistry at the Weinberg College of Arts and Sciences and co-corresponding author on the study.
This extra flexibility also causes the DNA to settle onto areas of the particles where they have more freedom to wiggle. For longer pieces of DNA, this tends to be at the nanoparticles’ vertices, or pointy ends. This arrangement of the DNA linkers causes the nanoparticles to glue together vertex-to-vertex. Shorter DNA molecules, on the other hand, tend to uniformly coat the particle surfaces, leading to structures that bind facet-to-facet.
“If the DNA linkers are flexible enough, we can control the types of structures that form by controlling the DNA length relative to the nanoparticle’s overall size,” said Kwanghwi Je, a PhD graduate from the Department of Chemical Engineering at the University of Michigan and the co-author of the study who performed simulations that predicted how the particles would arrange in space. “The beauty of this is that we can tune the length of the DNA linkers to guide the nanoparticles into specific structures.”
Controlling how the composite materials assemble into new structures could potentially yield materials with different properties, even when using the same building blocks.
The research was funded by the Air Force Office of Scientific Research, the US Department of Energy, the Simons Foundation, Northwestern University and the University of Michigan. The study used resources at NU’s Electron Probe Instrumentation Center, the NSF’s Extreme Science and Engineering Discovery Environment and U-M’s Advanced Research Computing. Sharon Glotzer is also the John Werner Cahn Distinguished University Professor of Engineering, the Stuart W. Churchill Collegiate Professor of Chemical Engineering and a professor of materials science and engineering, macromolecular science and engineering and physics.